38 auxiliary labels drug reference chart
General Chapter Prescription Container Labeling - USP-NF Estimated proposal PF: 46 (1) Background and objective: General Chapter <17> Prescription Container Labeling provides universal standards for the format, appearance, content, and language of prescription medication instructions to promote patient understanding and reduce medication errors. The Healthcare Quality and Safety Expert Committee ... Resources/Library - Pharmacy Techs Headquaters - for generic drugs. PDR. Physicians Desk Reference - commercially published compilation of manufacturers prescribing information, package inserts on prescription drugs - provides physicians with list of fully mandated information for writing prescriptions - widely available in libraries and bookstores. Auxiliary labels. For the X
PDF AUXILIARY LABEL - BC Cancer AUXILIARY LABEL . DATE: 1 May 2022. Page 1 of 14 . DRUG LABEL LABEL LABEL LABEL abemaciclib . abiraterone . acalabrutinib . acitretin . AFAtinib . alectinib . alitretinoin . anagrelide . AUXILIARY LABEL . DATE: 1 May 2022 Page 2 of 14 DRUG LABEL LABEL LABEL LABEL anastrozole . apalutamide .

Auxiliary labels drug reference chart
PDF NONSTERILE COMPOUNDING: BEYOND USE DATES and LABELING BEYOND USE DATES ... shortened in order to fit on the label. In the example above, Lid/Mlx/dip may not be used. If necessary, an auxiliary label stating complete drug names and amounts may be attached to the dispensing container in addition to the standard label. USP Chapter 795 requires the BUD and storage and handling information to be on the label. PDF Auxiliary Label Guiding Principles - BC Cancer developed the basic guiding principles for assigning auxiliary labels as follows: a. Auxiliary label information enhances but does not replace patient handouts or verbal counselling. b. A maximum of four auxiliary labels will be used due to container size limitation and to avoid alert fatigue. i. Exceptions: additional labels may be affixed to ... Safer dispensing labels for prescription medicines The use of standard dosing times, 'morning, noon, evening, bedtime', has been labelled the 'universal medication schedule'. 10, 12 The use of standard dosing times is feasible for most drugs and is less confusing, more informative and makes it easier for patients to consolidate multiple medicines into fewer dosing times throughout the day. 4, 9
Auxiliary labels drug reference chart. Labeling for Human Prescription Drug and Biological Products ... This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription drug and biological products under 21 CFR 201.56(d) and 201.57. Guidance Document: Labelling of Pharmaceutical Drugs for Human Use - Canada 3.8 Labelling of Professional Samples 3.9 Including International Information on Drug Package Labels Claims and Text Content 4.1 Misrepresentation of Classification 4.2 Absence of Ingredients 4.2.1 Sugar-free, Sucrose-free, Sweetener-free 4.2.2 Salt and Sodium-free 4.3 Absence of Side Effects 4.4 Side Effects and Placebo Comparisons Pharmacy Auxiliary Labels All labels measure 3/8" x 1 ½" and are brightly colored so they call attention to themselves. Because these labels have been in use for years, the graphics depicting what's being described are instantly recognized by your patients and practitioners. As always, if you do not find the item you are looking for, please call us at 800-523-8966! Veterinary Auxiliary Labels - Nev's Ink AUXILIARY LABEL, PRESCRIPTION CAN BE REFILLED - PAPER - PERMANENT - 3/8" X 1-1/2" - YELLOW W/BLACK - 1,000 - ROLL PAUXW-0013 $8.18 AUXILIARY LABEL, GENERIC DRUG HAS BEEN DISPENSED AT LOWER PR - PAPER - PERMANENT - 3/8" X 1-1/2" - RED W/BLACK - 1,000 - ROLL PAUXW-0012 $8.18
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning. PDF Revisions to APF24 Cautionary advisory labels Consult 'Drug interactions', page 111, a medicines information reference or the approved Product Information for more details. Label 12 revised explanatory notes Label 12 is recommended for medicines with the potential to cause CNS disturbances (e.g. dizziness, light-headedness, fatigue) and impair psychomotor performance. Auxiliary Medicinal Products in Clinical Trials information on the label shall be determined by the Member State concerned. The medicinal product may be labelled in several languages. 3.4 Safety reporting requirements for AxMPs This section applies to safety reporting requirement of adverse events suspected to be related to the AxMP only (= adverse reaction to AxMP). In case a suspicion of (or PDF Chapter 24 Medication Administration (Charting, Documentation and The ... Drug administration is the act in which a single dose of an identified drug is given to a patient. 2. Drugs shall be administered in compliance with all local, state and federal laws. ... the chart the following: a. The complaint or the symptom for which the drug was given. b. The dose, time, route of administration, and if appropriate the site ...
PDF Pharmaceutical Compounding and Dispensing - Ducopharm important additional auxiliary labels. The summary list given in Table 1.1 is to be used as a guide in the absence of any guidance from the offi cial pharmaceutical texts. It should be remembered that the information in this table is to be used only as a guide. Any information on additional labelling or expiry dates in the offi cial texts PDF BuSpar - Food and Drug Administration any drug's use can be identified only after several years of marketing. Information for Patients To assure safe and effective use of BuSpar, the following information and instructions should be given to patients: 1. Inform your physician about any medications, prescription or non-prescription, alcohol, or drugs that Oncology Nursing Society | ONF 2.3.3: Planned duration of treatment, schedule of treatment administration, drug names and supportive medications, drug-drug and drug-food interactions, and plan for missed doses. 29. 2.3.4: Potential long-term and short-term adverse effects of therapy, including infertility risks for appropriate patients. PDF Reference Guide for Pharmacy Technician Exam This reference guide is not intended as a substitute for the advice of a physician. Students or readers must consult their physicians about any existing problem. Do not use any information in this reference guide for any kind of self treatment. Do not administer any dose of mentioned drugs in this reference guide without consulting your physician.
Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ...
Guidelines for Prescription Labeling - The American Foundation for the ... Therefore, the Advisory Board recommends that pharmacies: Provide "duplicate labels" (prescription and auxiliary) printed in a minimum of 18-point type on paper stock. If pictograms are used, these should also be provided in "large print" format and high contrast (saturated black on white background).
Healthcare Auxiliary Labels | Nev's Ink AUXILIARY LABEL, REFILLED BY AUTHORITY OF YOUR DOCTOR - PAPER - PERMANENT - 3/8" X 1-1/2" - WHITE W/BLACK - 1,000 - ROLL PAUXW-0011 $8.18 AUXILIARY LABEL, GENERIC DRUG HAS BEEN DISPENSED AT LOWER PR - PAPER - PERMANENT - 3/8" X 1-1/2" - RED W/BLACK - 1,000 - ROLL PAUXW-0012 $8.18
PDF COMPLIANCE LABEL AND DRUG REFERENCE CHART • Ri 361 Steelcase Road West ... COMPLIANCE LABEL AND DRUG REFERENCE CHART • Ri 361 Steelcase Road West, unit Markham, Ontario L3R 3V8 Toronto 905-47 S -2500 Ton Free: :RTOIRE
Auxiliary labels - Student Doctor Network DDS Applicants How To Admissions Guides Dental Admissions Guide Occupational Therapy Admissions Guide How to Get Into ... Can someone assist in locating list of auxiliary labels (e.g. refrigerate, shake, take with food, etc.) that correspond to a specific drug? ... In my pharmacy practice lab there was a printout that had a list of drugs, and ...
PDF Lipitor (atorvastatin calcium) Label - Food and Drug Administration Drug therapy is recommended as an adjunct to diet when the r esponse to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In pateinst wtih CHD or mutlpi el rsikfactors for CHD L, IPITOR can be started smi utlaneousyl wtih deit.
PDF PTCB Study Guide - Fitzgerald Pharmacy Technician Class Drug-Drug Interactions The patient may be taking prescription or other nonprescription drugs that may interact with OTC medication. For example, drugs used for heartburn and acid indigestion interact with heart disease, blood pressure, and seizure disorders. Aspirin and Coumadin (warfarin) interact and could cause internal bleeding. 2.
Guidelines for Labeling Pharmaceutical & Healthcare Products Several important things to include on a pharmaceutical or healthcare product label: 3. Formatting Labels for FDA Approval. Your labels must be designed in the appropriate FDA format for your product's classification like OTC medications, oral contraceptives, combination products, etc. Click here for a list of labeling guides relating to drugs.
Use of Booklet Labels on Investigational Medicinal Products (IMPs) - ISPE A tear-off/peel-off label acts as a self-adhesive label used for documentation at the site. Retest Date Use period can be extended. Secondary Packaging Outer box/carton containing primary containers with IMP. Single Panel Label A label with just one layer; affixed directly onto the container containing one or more languages limited by size. Site
Pharmacy Tech. : Auxiliary Label Chart Flashcards | Quizlet Pharmacy Tech. : Auxiliary Label Chart STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by mattvah Terms in this set (43) ACE Inhibitor -May Cause Dizziness -Avoid Alcohol -Take on empty stomach ADHD -Avoid caffeine -May cause insomnia -May be habit-forming Analgesic -May cause dizziness -May cause drowsiness Antibiotic
PDF Red C stamp CHAPTER Labeling thePrescription Using the labels created from the corrected prescriptions in Chapter 2, begin the process of filling and labeling prescriptions. Steps: 1. Separate the paperwork according to patient. 2. Begin filling the prescriptions for one patient. 3. Double-check each label with the hard copy of the prescription. 4.
PDF Chapter 20 Labeling Medications and Expiration Dating and location, directions for use, and auxiliary labels c. Other labeling considerations: 1) The initials of the person preparing and verifying each compound 2) Placement of labels a) Affixed to containers so that they may be read while hanging b) Avoid covering manufacturer labeling containing drug name,
Coursework Hero - We provide solutions to students Simply kick back and relax. Coursework Hero will take good care of your essays and research papers, while you’re enjoying your day.
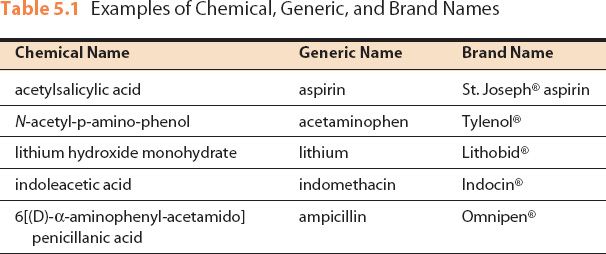










Post a Comment for "38 auxiliary labels drug reference chart"