40 legal requirements for dispensing labels uk
National standard for labelling dispensed medicines July 2021 Legislative requirements for the content of dispensed medicine labels are defined in the poisons and therapeutic goods regulations in each state and territory. Labelling requirements for Prescription Only Medicines (POM) and ... Apr 27, 2022 ... In practice the medicine will usually require over-labelling by a licensed unit leaving a space for the individual's name, date of dispensing, ...
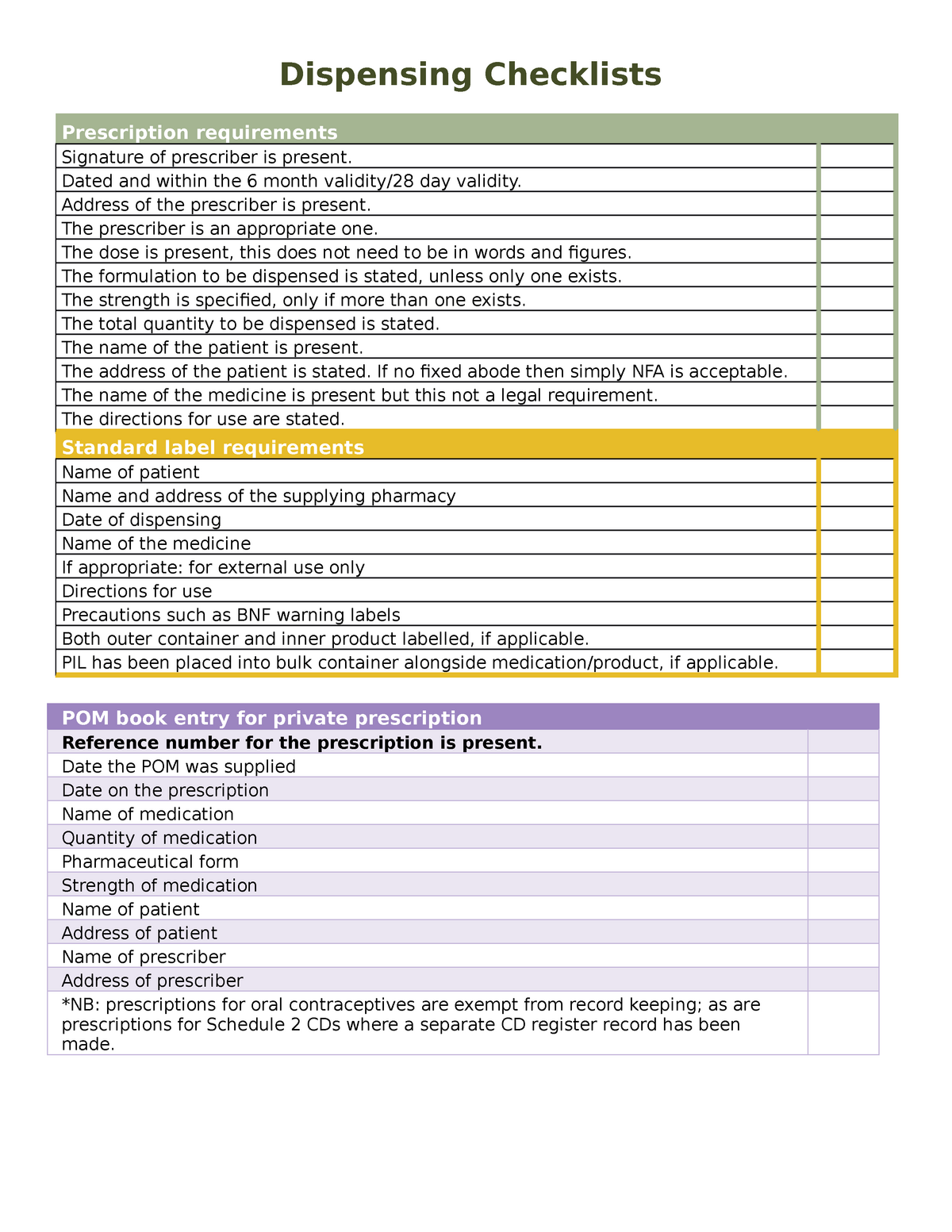
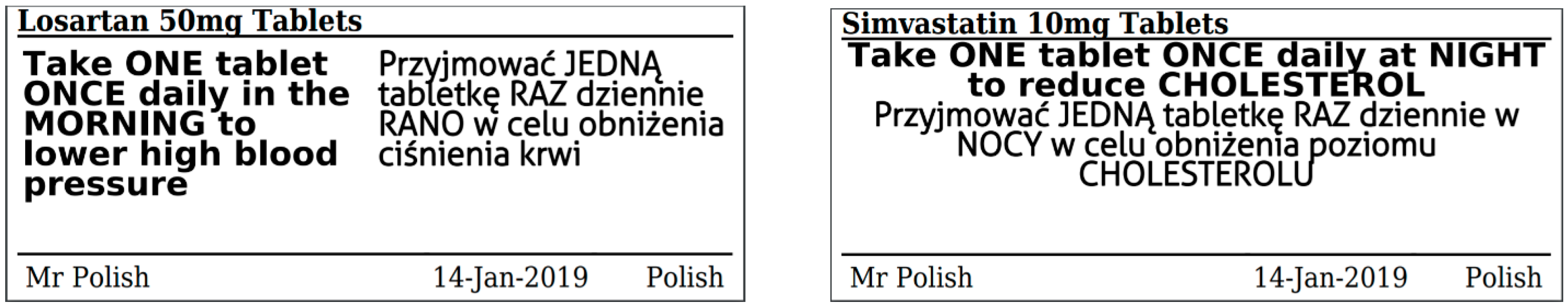
Supply of medicines | Medicines guidance - BNFC - NICE Labelling of prescribed medicines · name of the patient; · name and address of the supplying pharmacy; · date of dispensing; · name of the medicine; · directions for ...
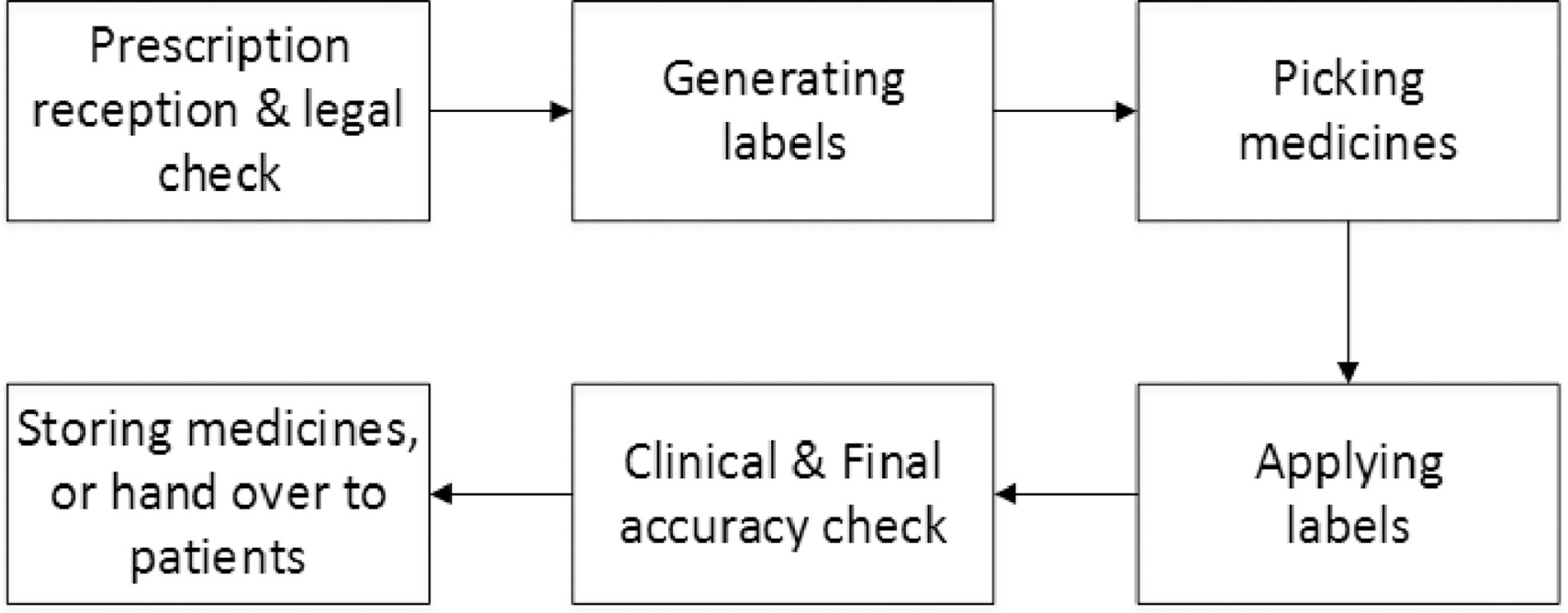
Legal requirements for dispensing labels uk
Best practice in the labelling and packaging of medicines - GOV.UK Dec 29, 2014 ... This guidance does not constitute a legal interpretation of the requirements on medicines labelling and packaging as set down within the Human ... Labelling of Dispensed Medicines - Drug Office Nov 18, 2021 ... All medicines dispensed by a pharmacy or medical practitioner should be labeled with the following essential information: name of patient; date ... Medicines: packaging, labelling and patient information leaflets Dec 18, 2014 ... Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional ...
Legal requirements for dispensing labels uk. Controlled Drug prescription forms and validity - PSNC Website Sep 13, 2022 ... Identity checks: There is a legal requirement for pharmacists to establish whether a person collecting a Schedule 2 CD is the patient, the ... The Medicines (Labelling) Amendment Regulations 1992 “Standard labelling requirements for containers and packages for medicinal products for human use ... 4A.—(1) Except where paragraph (2) or (3) of this regulation ... Best practice guidance on the labelling and packaging of medicines to the Medicines and Healthcare products Regulatory Agency (MHRA) as any ... Labelling must contain all elements required by Regulation 257 and Schedule. Optimising Dispensing Labels and Medicines Use Decision pathway · Relevant clinical specialism and competency, knowledge and experience of pharmacist · Availability of information, such as: Patient ...
Medicines: packaging, labelling and patient information leaflets Dec 18, 2014 ... Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional ... Labelling of Dispensed Medicines - Drug Office Nov 18, 2021 ... All medicines dispensed by a pharmacy or medical practitioner should be labeled with the following essential information: name of patient; date ... Best practice in the labelling and packaging of medicines - GOV.UK Dec 29, 2014 ... This guidance does not constitute a legal interpretation of the requirements on medicines labelling and packaging as set down within the Human ...




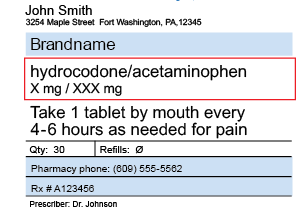




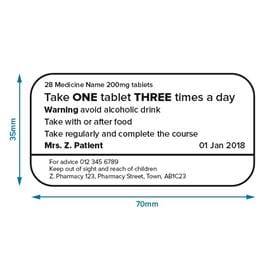













Post a Comment for "40 legal requirements for dispensing labels uk"